
What to consider
Intermittent Injections on Demand
There are two important aspects to Apomorphine injections for Parkinson’s patient (Trenkwalder et al., 2015):
- The speed of drug effect onset after intermittent Apomorphine injection, making it desirable for the patient who has a predictable but delayed response to levodopa of 15-30 min or longer
- The reliability of its effect at recovering an “On” state (as compared to the effects of oral dosing with levodopa).
- The short half-life of Apomorphine induces a response of about 45-60 min, which does not generally interfere with the basal drug regimen, but fills the gaps in motor functioning.
Subcutaneous Apomorphine injections provide a rapid and reliable “On” state for patients with morning akinesia, as a result of avoiding gastrointestinal delivery and absorption. Reasons for considering s.c. Apomorphine bolus injections on demand:
- To treat morning problems like akinesia and dystonia
- Problems with gastric emptying (gastroparesis)
- When rapid and reliable relief is required during both predictable and non-predictable “Off” periods
- To bridge delayed “On”
- Prevent from dose failures
- To treat non-motoric “Off” (e.g. pain)
Continuous Pump Infusion
Development of motor fluctuations and dyskinesia characterizes the transition from the early to the moderate and advanced Parkinson’s stage.
Apomorphine determines more continuous striatal dopamine receptors stimulation resulting in significant reduction of “off”-time and dyskinesia, particularly peak-dose. Early intervention ideally would target patients as soon as motor complications begin rather than at late stage. Preliminary evidence from early pump treatment suggests that this is feasible (Antonini & Jost, 2018).
The primary treatment goal is to reduce the intensity and duration of “off” periods. Continuous and constant dopaminergic stimulation with subcutaneous infusion mimics best the effect of dopamine in the brain. The therapy may reduce motor complications and dyskinesias.
Dacepton® pump infusions can reduce length and intensity of „off“ periods and improve motor complications which consequently enables a better symptomatic outcome in PD than previously insufficiently reached with optimized oral anti-Parkinson medication. Dacepton® continuous infusion therapy may also reduce the intake of oral PD medication enabling patients to enjoy life more independently.
Apomorphine Test
The required dose of Apomorphine is ascertained by an Apomorphine response (challenge) test. The challenge is necessary to determine that the patient has a positive response to Apomorphine, to establish the dose required to produce this response, and to identify their susceptibility to potential side effects such as postural hypotension, hallucinations, nausea or excessive somnolence.
Usually three days prior to the test the patient will be asked to take an anti-emetic (domperidone, three times a day). The test will be done with small injection needles. At each dose level the patient will be asked to attempt a number of motor assessments, including standing, walking or finger tapping. The test procedure may be as followed below (Hagell et al., 2014).
Apomorphine Test Scheme
Preparation
- Premedication with domperidone: 3x daily for 48 to 72 hours before the Apomorphine test and 1 hour before starting the test.
- Discontinuation of anti-parkinsonian medication
A lower dosage and shorter interval of the premedication (e.g. 10mg domperidone 3x daily for 24 hours) is adequate in most cases (Pietz et al., 1998), but a higher dosage may reduce the risk of peripheral dopaminergic side effects (Rascol et al., 1990). If domperidone is not available, 200 to 300mg trimethobenzamide may be given 3x daily (Bowron, 2004).
Dosage Scheme
Option A
Starting dosage: 1.0mg Apomorphine s.c.
Stepwise increase of the dosage by 1.0 to 1.5mg
to a maximum dosage of 10mg every 45 minutes.
Option B
Starting dosage: 1.5mg Apomorphine s.c.
Stepwise increase of the dosage by 1.0 to 2.0mg
to a maximum dosage of 10mg every 45 minutes.
Option C
(sometimes practiced):
Single dosage of 3mg Apomorphine s.c.
Shorter dosage intervals (e.g. 30 minutes) after second assessment after injection and/or higher dosage steps may be considered (Lees et al., 1998).
Criteria for Significant Response
At least two of the following criterias should be present:
- UPDRS part III: motor assessment: = 20% improvement versus baseline
- Hand-arm-movement between two distant points of 30cm: = 15% improvement versus baseline
- 2x7m walk: = 20% improvement versus baseline
UPDRS part III shows the best sensitivity and specificity of dopaminergic response when a reduction between 15% and 20% versus condition without medication is monitored (Rossi et al., 2000). A threshold of 15% for hand-arm-movements between two distant points in a given period of time has turned out to be an adequate assessment of the dopaminergic response of bradykinesia (van Hilten et al., 1997). Walking in a given period of time has a good correlation with other clinical assessments like e.g. the motor assessment using the UPDRS (Martínez-Martín et al.,1997).
Testing Procedure
- Baseline-assessment without medication UPDRS part III, motor assessment (Fahn et al., 1987), hand-arm-movement between two distant points of 30cm: counting of cycles within 20 seconds (Defer et al., 1999); 7m walk, turn around, walk back: measuring time in seconds, number of steps, including turns;
if the patient is not able to walk 7m within 90 seconds measuring the distance and the number of steps within 90 seconds (Hagell, 2000). - Injection of Apomorphine s.c. (abdominal wall) according to dosage scheme A or B
- Assessment of the motor function (UPDRS part III, hand-arm-movements, 2x7m walk) 20 minutes after Apomorphine s.c. application)
Testing Results
- After significant motor response (see criteria): stop test
- After negative or insignificant motor response: repeat the test (step 2 and 3 of the testing procedure if side effects are not too severe after 45 minutes)
- If side effects after 1.5mg Apomorphine s.c. are too severe: repeat the test at a dosage of 1.0mg Apomorphine s.c.
- Option C: If side effects after 3.0mg Apomorphine s.c. are too severe (despite motor response): stop test
Quick Titration
When performed for dose-finding purposes in preparation for a planned trial of intermittent s.c. Apomorphine therapy, a quicker testing protocol has successfully been tried in Sweden during the past few years. According to this protocol, s.c. Apomorphine injections are administered with brief intervals (15 minutes) until a satisfactory effect is gained or unacceptable side effects occur.
This protocol (see table is based on the pharmacokinetics of Apomorphine and takes advantage of residual plasma Apomorphine levels by building up the bioavailable dose. Pulse and blood pressure (supine and standing) and motor response should be monitored every 10-12 minutes (Hagell et al., 2020).
Route of Administration
Dacepton® should be initiated in the controlled environment of a specialist clinic. The patient should be supervised by a physician experienced in the treatment of Parkinson’s disease (e.g. neurologist). The patient’s treatment with levodopa, with or without dopamine agonists, should be optimised before starting treatment with Dacepton®. It is essential that the patient is established on Domperidone, usually 20 mg three times daily for at least two days prior to initiation of therapy. Once treatment has been established Domperidone therapy may be gradually reduced in some patients but successfully eliminated only in a few, without any vomiting or hypotension.
Dacepton® may be administered by either intermittent s.c. injection at the beginning of “off“ phases, or by continuous s.c. infusion. Dacepton® 10 mg/ml is for subcutaneous use by intermittent bolus injection. Dacepton® 10 mg/ml may also be administered as a continuous subcutaneous infusion by minipump and/or syringe-driver (SmPC, in current version).
Dacepton® 5 mg/ml solution for infusion is a pre-diluted vial intended for use without dilution for subcutaneous use and to be administered as a continuous subcutaneous infusion by minipump and/or syringe-driver. It is not intended to be used for intermittent injection (SmPC, in current version).
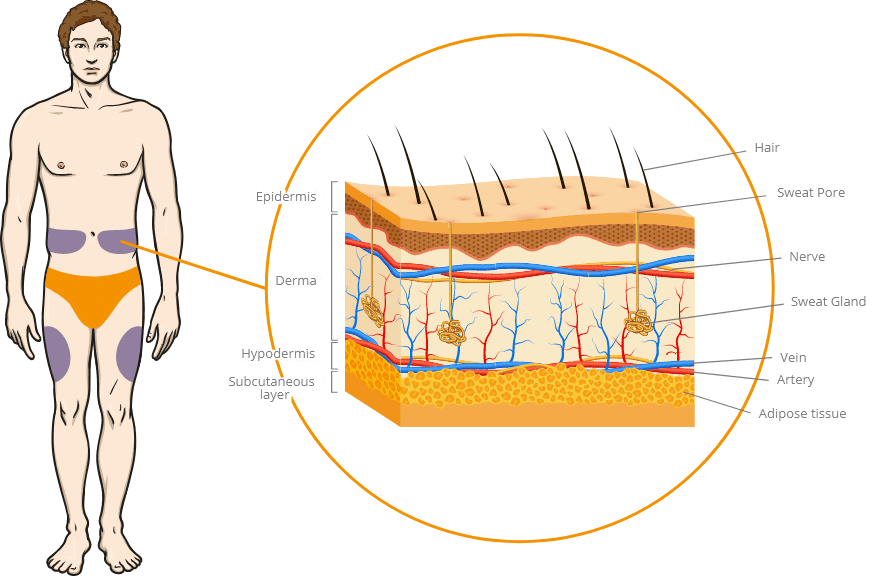
Injection sites and layer of skin
Intermittent injection of Dacepton®
Intermittent s.c. injections are used as a strategy on demand for disabling refractory ”off” periods, in patients already treated with an optimized oral anti-Parkinson therapy (Lees, 2002).
Delays in turning “On” reflect a delay in the absorption of Levodopa and a subsequent delay in crossing the blood–brain barrier. This may be a result of delayed gastric emptying (gastroparesis) or presence of intestinal pro¬tein that competes with L-dopa absorption (Stocchi, et al., 2008).
Prolonged morning akinesia, during which time patients remain in an “Off” state in advance of a therapeutic response from their first morning Levodopa dose, is a common clinical manifestation in patients with delayed “On” (Isaacson, et al., 2016).
Selection criteria for Apomorphine intermittent therapy
- Symptom response to levodopa
- Neuropsychatric and cognitive status
- Diary showing repeated “on-off” periods even with optimized oral medication
- Care-giver and specialized PD nurse to support application
In addition to the selection criteria, observation of the ECG, blood count (RBC, WBC) and renal function is necessary. It is also relevant whether orthostatic hypotension occurs.
The D-mine® Pen for Dacepton® 10 mg/ml in 3 ml cartridges
The reusable D-mine® Pen injector is a high quality pen developed by EVER Pharma for intermittent bolus injections with Dacepton®.
The automatic delivery-system enables low and constant injection force for PD patients with motoric impairment. Possible dose correction and last dose stop guarantee safe comfort.
The D-mine® Pen injection may deliver a controlled “on” period for patients and has therefore a positive impact on daily activities!

Considerations before administering intermittent Apomorphine therapy
Once the appropriate dose is determined a single subcutaneous injection may be given into the lower abdomen or outer thigh at the first signs of an “off” episode. It cannot be excluded that absorption may differ with different injection sites within a single individual. Accordingly, the patient should then be observed for the next hour to assess the quality of their response to treatment. Alterations in dosage may be made according to the patient’s response.
The optimal dosage of Apomorphine hydrochloride varies between individuals but, once established, remains relatively constant for each patient.
It is recommended that the total daily dose of Apomorphine hydrochloride should not exceed 100 mg and that individual bolus injections should not exceed 10 mg per hour (Hagell, et al., 2014; SmPC, in current version).
How to set up the D-mine® Pen
How to use the D-mine® Pen
Continuous Infusion of Dacepton®
Patients who have shown a good “on” period response during the initiation stage, but whose overall control remains unsatisfactory using intermittent injections, or who require many and frequent injections (more than 10 per day), may be commenced on or transferred to continuous subcutaneous infusion by minipump and/or syringe-driver.
Continuous infusion is started at a rate of 1 mg Apomorphine hydrochloride (0.1 ml) per hour then increased according to the individual response. Increases in the infusion rate should not exceed 0.5 mg per hour at intervals of not less than 4 hours. Hourly infusion rates may range between 1 mg and 4 mg (0.1 ml and 0.4 ml), equivalent to 0.015 – 0.06 mg/kg/hour. Infusions should run for waking hours only. Unless the patient is experiencing severe night-time problems, 24 hour infusions are not advised.
It is recommended that the total daily dose of Apomorphine hydrochloride should not exceed 100 mg. Patients may need to supplement their continuous infusion with intermittent bolus boosts via the pump system as necessary, and as directed by their physician. A reduction in dosage of other dopamine agonists may be considered during continuous infusion (SmPC, in current version).
The D-mine® Pump for Dacepton® 5 mg/ml in 20 ml vials
An intuitive easy to use mg-based volumetric infusion pump system specifically developed for patients with Parkinson‘s Disease.
Automatic filling ensures safe medication handling and up to 5 different rates are programmable. With a few simple steps user training times are reduced. The D-mine® Pump supports greater self-reliance and mobility for your Parkinson’s patients!
Further examples for infusion syringe drivers:

How to set up the D-mine® Pump
How to use the D-mine® Pump

Download
Find the SmPC, Instructions for Use of the Medical Devices and other Supportive Documents in the Download Area.

Congresses
Find upcoming congresses and events in this area.